The Black Ink Team’s Guide To How Batteries Work
by Black Ink Team
When we aren’t busy producing a powerful CRM, we like to educate ourselves and others about the products our platform is designed to help professionals sell. In doing so, we hope to make it easier for salespeople to pitch said products and for customers to see their value. That being said, here’s a guide to how batteries power battery-powered equipment/machinery.
Although they power electrical devices, batteries do not store electrical energy – they really store chemical energy. To release and use said energy, all one must do is simply use the battery to complete a circuit.
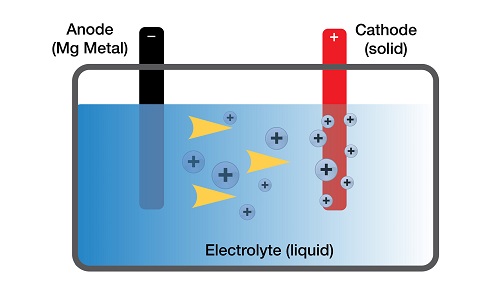
Inside a battery there are three main parts: an anode, a cathode, and an electrolyte. Anodes and cathodes are both also called electrodes, although one is negative and the other is positive (respectively). The electrolyte is there to serve as a buffer between the two electrodes; if it wasn’t there, they would immediately short-circuit and zero energy would ever leave the battery. Another term for a battery (when it isn’t being charged) is ‘electrochemical cell.’
When a rechargeable battery is being charged, the polarities of its electrodes are reversed – the cathode becomes negative and the anode becomes positive. This makes it an ‘electrolytic cell,’ because it is converting electrical energy into chemical energy (as opposed to converting chemical energy into electrical energy).
The specific type of chemical reaction that occurs inside batteries to produce electrical energy (when they are being used to power something), or to accumulate stored chemical energy (when they are being charged), is a ‘redox’ reaction, which is short for ‘oxidation-reduction.’ In a redox reaction, electrons are passed off between atoms. ‘Oxidizing’ refers to atoms losing electrons, and ‘reducing’ refers to atoms gaining electrons – yes, you are correct, that second part is confusing. What makes it make sense is that the charge of an electron is negative, so therefore when an atom gains an electron its charge reduces.
So, what happens exactly when you initially turn on something that is battery-powered – say, a battery-powered leaf blower – you may ask? After you flip its switch, a circuit encompassing a battery gets completed, which allows for the flow of electrons. On the anode, the battery’s negative ‘terminal,’ a redox reaction occurs, thus releasing electrons through the circuit and ions into the electrolyte simultaneously. The ions pass through the electrolyte to the other side of the battery, where the electrons are being channeled back into the battery via a redox reaction on the cathode. While travelling through the circuit, the emancipated electrons power a motor – which turns a fan, which blows air, which moves leaves. Once they are back in the battery, on the cathode side, the electrons bond with the ions.
You’re probably now wondering: why, if the electrons go back into the battery at the end of the circuit, do batteries ever need to be recharged? For a comprehensive answer to that question, one article alone couldn’t possibly suffice, but the short explanation we will give here is that yes, the electrons do end up back in the battery, but the arrangements that they end up in ultimately makes them less available to be transferred.
